Anticancer, Antioxidant and Antimicrobial Activity of Annonaceae Family
- Research article
- Open Admission
- Published:
Pharmacologically agile flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl
BMC Complementary and Culling Medicine volume xvi, Article number:460 (2016) Cite this article
Abstract
Background
Cassia angustifolia Vahl. (unremarkably known as senna makkai or cassia senna), native to Saudi arabia, Egypt, Yemen and also extensively cultivated in Pakistan, is a medicinal herb used traditionally to cure number of diseases like liver diseases, constipation, typhoid, cholera etc. This report was conducted to evaluate the in-vitro antimicrobial, antioxidant and anticancer assays and phytochemical constituents of aqueous and organic extracts of C. angustifolia leaves.
Methods
The antimicrobial activities of C. angustifolia aqueous and organic (methanol, ethanol, acetone, ethyl acetate) extracts were investigated by the disk improvidence method. These extracts were further evaluated for antioxidant potential by the DPPH radical scavenging assay. Anticancer activities of the extracts were adamant past the MTT colorimetric assay. The total phenolic and flavonoid contents of C. angustifolia extracts were evaluated by the Folin-Ciocalteu method and aluminum chloride colorimetric assay, respectively. The structures of the bioactive compounds were elucidated by NMR and ESI-MS spectrometry.
Results
Bioactivity-guided screening of C. angustifolia extracts, led to the isolation and identification of three flavonoids quercimeritrin (1), scutellarein (ii), and rutin (three) reported for the first time from this plant, showed meaning anticancer activity confronting MCF-vii (IC50, 4.0 μg/μL), HeLa (ICfifty, 5.45 μg/μL), Hep2 (IC50, seven.28 μg/μL) and low cytotoxicity confronting HCEC (IC50, 21.09 μg/μL). Significant antioxidant activity was observed with IC50 2.41 μg/mL confronting DPPH radical. Moreover, C. angustifolia extracts take the potential to inhibit microbial growth of Eastward. cloacae, P. aeruginosa, S. mercescens and S. typhi.
Conclusion
C. angustifolia extracts revealed the presence of quercimeritrin (1), scutellarein (ii), and rutin (3), all known to have useful bioactivities including antimicrobial, antioxidant and anticancer activities.
Groundwork
In contempo years, there has been an alarming increase in the antibody resistance to a broad range of human pathogenic bacterial and fungal strains which contribute to the recurrence of infectious diseases. Urinary tract, bloodstream, and respiratory organs are some of the mutual sites of infections caused by antibiotic resistance microbes throughout the world [1]. Multidrug-resistant bacterial and fungal strains are the main cause of infirmary acquired infections which reduce the efficacy of drugs and are ultimately responsible for treatment failure [two]. This situation has created a need to observe more effective drugs. Natural products from microorganisms have been the primary source of antibiotics, and with the increasing credence of herbal medicines, the screening of medicinal plants for new agile compounds has become a very important source of novel antibiotics [three].
Extensive researches on medicinal plants have also indicated that they are good sources of antioxidants [four–half dozen]. They are involved in free radical scavenging activities which contribute to the protection from oxidative stress caused past the overproduction of costless radicals and reactive oxygen species [7]. These are produced equally byproducts of diverse biochemical and physiological processes in the human being body [8]. Biomolecular torso systems such equally lipids, DNA, RNA and proteins are adversely affected by the oxidative stress which eventually leads to human chronic diseases such every bit Alzheimer'southward, cardiovascular, atherosclerosis, cancer, stroke, fibrosis, aging and diabetes [nine]. According to many inquiry reports, the consumption of medicinal plants either in the class of raw extracts or chemical constituents is largely associated with lower run a risk of degenerative diseases caused by oxidative stress considering they contain antioxidants such as phenolics, flavonoids, vitamins and carotenoids [10]. Phenolic compounds such every bit phenolic acids and flavonoids are reported to exist involved in various biochemical activities like antioxidant, antimicrobial, antithrombotic, antiartherogenic, anti-inflammatory, anticarcinogenic and antimutagenic [11]. Natural antioxidants of institute origin are usually more potent and benign than synthetic antioxidants such as propylgallate (PG), butylated hydroxytoluene (BHT), t-butylhydroxytoluene (TBH) and butylated hydroxyanisole (BHA) [12]. It was also reported that constructed antioxidants were the cause of carcinogenesis and liver impairment in laboratory animals [13]. Thus there is a demand to explore and develop antioxidants of natural origin with greater efficacy and fewer side effects.
Cancer is the second largest cause of death worldwide [fourteen]. Although nifty advancements have been made in the treatment and control of cancer progression, meaning deficiencies and room for improvement remains. A number of undesired side effects sometimes occur during chemotherapy. Natural therapies, such every bit the use of constitute-derived products in cancer treatment, may reduce adverse side effects. There are many natural products including phytochemicals and dietary compounds from vegetables, plants, spices and herbs that accept been used for the treatment of cancer throughout history due to their prophylactic, low toxicity, and general availability [15].
C. angustifolia Vahl. is a traditional medicinal plant belonging to the family Caesalpiniaceae. It is unremarkably known equally senna makkai or cassia senna. C. angustifolia is native to Saudi Arabia, Egypt, and Republic of yemen. It is a rapid-growing shrub five–8 thou tall, extensively cultivated for its fruit and leaves in hot barren areas of Pakistan [16]. This plant is recognized in British and U.s. pharmacopoeias [17]. The leaves and pods of C. angustifolia are used in the form of a decoction powder for intestinal worms as an anti-helmenthic. It is as well widely used as an anti-pyretic in typhoid, splenic enlargements, cholera, laxative, anemia, toxicity and genotoxicity caused by Escherichia coli [sixteen].
Worldwide consumption of C. angustifolia as a folk medicine against various ailments and the studies reported in the literature demand further research to notice the compounds responsible for its bioactivities. This enquiry study was conducted to investigate the antibacterial, antifungal, antioxidant, and anticancer potentials of aqueous as well as organic extracts of C. angustifolia. They were also subjected to phytochemical screening to make up one's mind the presence of secondary metabolites and bioactive compounds.
Methods
Formulation of plant crude extracts
C. angustifolia seed powder were bought from a local herb shop in Islamabad and is identified past taxonomist Dr. Muhammad Qasim Hayat, ASAB (Atta-ur-Rahman School of Applied Biosciences), NUST (National University of Sciences and Applied science), Islamabad. It was identified by comparison with the voucher specimen no P03088812 of Herbier museum Paris (http://mediaphoto.mnhn.fr/media/1441330963480e0yHgeqQ0CSk9mGW). The local botanical description of C. angustifolia is likewise available at flora of Islamic republic of pakistan (http://world wide web.tropicos.org/Name/13028414?projectid=32) and original constitute material was kept at MPRL (Medicinal Establish Inquiry Laboratory), ASAB, NUST for futurity references. Extracts were formulated past maceration. The finely footing powder (mesh size fifty = 0.297 mm) was subjected to aqueous and organic solvents (methanol, ethanol, acetone, and ethyl acetate) separately in flasks with the ratio 1:10 and placed in the night at 37 °C for 3 days with intermittent shaking. Centrifugation was performed for 15 min at 2000 rpm. Supernatants were filtered with Whatman filter newspaper no.one. The filtrate was transferred to a round-bottom flask and the solvent was rotary evaporated. The dry extract was stored at iv °C.
Bacterial and fungal strains
Acinetobacter junii IARS2, Serratia mercescens IARS6, Enterobacter cloacae IARS7, Pseudomonas aeroginosa IARS8 and Salmonella typhi ATCC 14079 were used in antibacterial assays. Glycerol stocks of all the bacterial strains were maintained in controlled conditions and subcultured on Mueller Hinton agar for 24 h before antibacterial assay. The fungal strain Candida albicans was obtained from the Establish of Biotechnology and Genetic Applied science (IBGE), Abdul Qadeer Khan Enquiry Laboratory (KRL) Hospital, Islamabad. It was maintained at 37 °C on Sabouraud dextrose agar.
Antimicrobial assay
Organic and aqueous extracts of C. angustifolia were screened for antibacterial and antifungal potential past disk diffusion analysis as previously reported [18].
Antioxidant assay
The antioxidant activities of the organic and aqueous extracts of C. angustifolia and gallic acid were evaluated with the DPPH method as previously described [19]. Antioxidant potential of C. angustifolia extracts were analyzed and mentioned as ICfifty, which was calculated from the calibration curve of the standards past using MS Excel 2010.
Anticancer assay
Cell culture
The HeLa was provided by HBV (Hepatitis B Virus) lab, ASAB, NUST. Hep2, MCF-7, and HCEC were provided by IBGE, KRL Hospital, Islamabad. All the cell lines were grown in RPMI-1640 media which contained 10 % FBS. All cells were maintained at 37 °C in a humidified atmosphere of 5 % CO2.
MTT analysis
Anticancer assay of C. angustifolia on Hep2, HeLa, MCF-7, and HCEC were carried out past the MTT colorimetric assay as employed earlier [20]. The anticancer activities of each tested extract were presented as IC50, which was calculated past percentage cell death at 100 μg/μL, 150 μg/μL, 200 μg/μL, 250 μg/μL using MS excel 2010.
Qualitative phytochemical screening for secondary metabolites
C. angustifolia extracts were phytochemically screened for the presence of secondary metabolites. Qualitative phytochemical analyses for the presence of steroids, alkaloids, tannins including phlobatannins, monoterpenes, flavonoids, coumarins, cardiac glycosides, saponins, diterpenes, anthraquinones, and phenols were carried out by standard protocols [21–23].
Determination of total phenolic contents
Total phenolic contents of the organic and aqueous extracts of C. angustifolia were determined past the Folin-Ciocalteu method [24].
Conclusion of full flavonoid contents
The total flavonoid contents of C. angustifolia extracts were determined by the aluminum chloride colorimetric method [19].
Isolation of agile compounds
The C. angustifolia extracts were submitted to HPLC-MS analysis for screening of active compounds. A Shimadzu preparative HPLC, equipped with an LC-20 Advertising pump (the make-up pump), two LC-8A pumps (the slope pumps), an SPD-20A UV detector and a CTC analytics PAL sample injector and fraction collector were used for the isolation of compounds. The fractions were collected based on UV @ 214 nm. The column used was a Phenomenex Gemini-NX C18 (thirty × 50 mm i.d., 5 μm particle size, 110 A). The injection volumes were 800 to 1200 μL. The gradient was 5 to lxx % acetonitrile over 25 min with a period rate of 35 mL/min. The modifier was 0.1 % formic acid, used primarily for ionization.
Identification of active compounds
The structural identifications of isolated compounds were carried out by 1H NMR analysis. 1H NMR data were obtained by using a Bruker AVANCE 3 400 MHz. NMR spectrometer equipped with a PA BBO 400S1 probe and a sample jet autosampler.
Statistical assay
All the experiments were carried out in triplicate. Data were presented as mean ± SD. T-Test was performed to determine statistical significance. Microsoft Excel 2010 was used for the statistical and graphical evaluations.
Results
Antimicrobial action
The antibacterial activities of different extracts of C. angustifolia and controls are shown in Tables 1 and 2. All the tested pathogenic bacterial strains were sensitive to C. angustifolia extracts. Extracts of C. angustifolia showed variable degrees of bactericidal activity, with inhibitory effects of methanol extracts being observed confronting all of the selected bacterial strains. The highest bactericidal action of the ethyl acetate extract was recorded against S. mercescens, with a 10.v ± 0.76 mm zone of inhibition at one.25 mg/mL. Information technology was observed that A. junni, E. cloacae and P. aeroginosa were resistant to aqueous extract. The ethanol extract showed no antibacterial activeness confronting E. faecalis and P. aeruginosa, while it showed its best antibacterial activity against S. mercescens with a 9.0 ± 0.50 mm zone of inhibition at 1.25 mg/mL. A. junii, S. mercescens, Eastward. cloacae, and S. typhi were sensitive to acetone excerpt. Tigecycline, amikacin and cefepime (5 μg/mL) were used every bit standard antibiotic drugs (Table 2).
The antifungal potential of the extracts were measured in terms of clear zone of inhibition of fungal growth (Table 1). All the tested C. angustifolia extracts had meaning antifungal activity. The methanol excerpt exhibited the highest antifungal activity with a zone of inhibition of 12 mm at 10 mg/mL as compared to the standard amikacin drug which is sensitive to fungal growth. The aqueous extract showed no pregnant antifungal activity while the ethanol and ethyl acetate extracts showed moderate antifungal action with zones of inhibition of 11 and 10 mm, respectively.
Antioxidant assay
The antioxidant activities of the aqueous and organic extracts of C. angustifolia and gallic acid were evaluated past the free radical DPPH scavenging test on the ground of IC50 values (Tabular array 3). ICl values are the inhibitory concentrations required for 50 % scavenging of DPPH gratis radicals. The smaller the IC50 values, the college the antioxidant potential of the plant constituents. The absorbance values of different extracts of C. angustifolia and standards were measured at the wavelength 517 nm. The resulting absorbance of the extracts give pct scavenging of DPPH free radicals. All the extracts have dose dependent antioxidant activities, i.e., the scavenging activities of the extracts increased with the respective increment in the concentrations (Fig. 1). According to the results, all the extracts accept potential antioxidant activities. The ethanol extracts showroom maximum DPPH free radical scavenging activeness (93 %) at a concentration of 500 μg/mL with an IC50 value of 2.41 ± 0.02 μg/mL, whereas the aqueous excerpt showed poor DPPH scavenging activeness (68 %) at 500 μg/mL with IC50 values of 3.03 ± 0.04 μg/mL. The other C. angustifolia extracts showed moderate DPPH scavenging activities (Fig. 1).
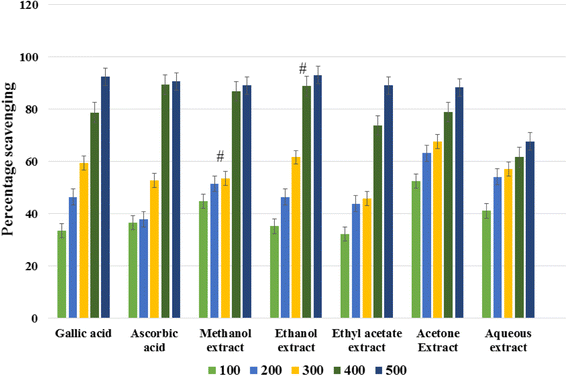
Graph showing DPPH radical scavenging action of unlike extracts of C. angustifolia. Each point represents the hateful of 3 experiments. Information are expressed as mean ± standard departure. (#) indicated values are non significant at P < 0. 05
Anticancer assay
The cytotoxic studies for C. angustifolia extracts employed the MTT colorimetric method. The C. angustifolia aqueous, methanol, ethanol, acetone and ethyl acetate extracts were investigated beginning fourth dimension for their anticancer potential against Hep2, HeLa, MCF-7 and normal HCEC jail cell lines. Only the ethanol extract exhibited anticancer action, with 28 % death in Hep2 cells with an IC50 of 7.28 μg/μL. Methanol and ethanol extracts exhibited 33 and 23 % cell decease in HeLa jail cell lines and 43 and 23 % cell expiry in MCF-7 cell lines (Tabular array iv). The IC50 values are used to detect the dominance of drugs, lower ICl values mean more than potent the drugs. It was observed that ICl value of methanol extract is 5.45 μg/μL against HeLa cells and 4 μg/μL confronting MCF-7 cells which are far less than the ICl value of standard anticancer drug taxol 6.07 μg/μL and tamoxifen 6.iv μg/μL. C. angustifolia methanol and ethanol extracts were further examined for cytotoxicity confronting the normal cell line to answer whether it is selective towards cancerous cells. For this, normal HCEC cells were incubated with different concentrations of methanol and ethanol extracts (100, 150, 200 and 250 μg/μL) to analyze prison cell viability. The information showed that HCEC cells were unaffected by exposure to methanol and ethanol extracts. Information technology showed ca. 100 % prison cell viability against different concentrations of methanol and ethanol extract. These results showed that methanol and ethanol extracts have the potential to inhibit the proliferation of Hep2, HeLa, and MCF-vii cells. These cells were more sensitive to inhibition by C. angustifolia extract than normal HCEC prison cell lines.
Phytochemical screening
Qualitative phytochemical screening of unlike extracts of C. angustifolia revealed the presence of steroids, alkaloids, tannins including phlobatannins, monoterpenes, flavonoids, coumarins, cardiac glycosides, saponins, diterpenes, anthraquinones, and phenols which contribute to the antimicrobial, antioxidant and anticancer activities of the found (Tabular array 5).
Total phenolic contents
Full phenolic contents in the aqueous and organic extracts of C. angustifolia were evaluated past plotting a standard bend using different concentrations of GAE (Gallic acid equivalent) with their respective absorbance at 700 nm. The linear regression equation [y =0.504× (R2 = 0.991)] for the scale curve was used to evaluate the total amount of phenolic contents present in each extract which was expressed as mg of GAE/one thousand (Table 6). The analysis showed that a considerable amount of phenolic contents were present in organic and aqueous extracts of C. angustifolia ranging from 0.535 ± 0.002 to 2.328 ± 0.003 mg of GAE/grand of extract. The methanol and ethanol extracts have the well-nigh phenolic contents followed in order by the ethyl acetate, acetone and aqueous extracts.
Total flavonoid contents
The total flavonoid contents were determined by plotting a standard curve using different concentrations of QE (Quercetin equivalent) with their absorbance at 510 nm. The linear regression equation [y =0.040× (R2 = 0.975)] of the calibration bend was used to evaluate the total amount of flavonoid contents present in each extract which is expressed as mg of quercetin equivalents per gram QE/gm (Table 6). Methanol extracts of C. angustifolia showed higher content of flavonoids than the other solvent extracts.
HPLC analysis
Bioactivity-guided screening of C. angustifolia methanol, ethanol and ethyl acetate extracts past HPLC-MS revealed the presence of 3 bioactive compounds: quercimeritrin (one), scutellarein (two), and rutin (3).
Quercimeritrin (1)
C21HtwentyO12, yellow amorphous powder; oneH NMR (400 MHz, CD3OD) δ: iii.48-3.98 (6H, grand, H-2", H-3", H-iv", Ha-5", Hb-5"), 5.25 (1H, d, J = 8 Hz, H-one"), 6.25 (1H, d, J = 2 Hz, H-8), 6.48 (1H, d, J = 2 Hz, H-6), half dozen.86 (1H, d, J = 8.5 Hz, H-5'), 7.63 (1H, dd, J = 8.5, 2.5 Hz, H-half-dozen'), 7.67 (1H, d, J = two.0 Hz, H-2'). ESIMS chiliad/z 464.38 [M]+, 465.38 [Yard + H]+, 487.38 [M + Na] +, 463.37 [M-H]−.
Scutellarein (ii)
C15H10O6, reddish-brown crystals; 1H NMR (400 MHz, CDiiiOD) δ: 6.22 (1H, s, H-3), 6.76 (2H, d, J = 8, H-3',5'), 7.09 (2H, d, J = 8, H-ii',vi'), seven.11 (1H, s, H-eight). ESIMS m/z 286.15 [Grand]+, 287.15 [G + H]+, 309.15 [Grand + Na] +, 285.14 [M-H]−.
Rutin (3)
C27H30Oxvi, yellow-brown crytals; 1H NMR (400 MHz, CDthreeOD) δ: 1.15 (3H, d, J = vi, H-vi"'), 3.27 (1H, thou, H-four"'), iii.45 (1H, one thousand, H-5"'), 3.56 (1H, dd, J = 9.5/3.5 Hz, H-3'"), 3.65 (1H, dd, J = iii.5/1.v, H-two'"), 3.26-3.53 (6H, m, H-2", H-3", H-4", Ha-5", Hb-5"), 5.13 (1H, d, J = seven.8 Hz, H-1"), 6.24 (1H, d, J = i.8 Hz, H-six), 6.43 (1H, d, J = 2.ii Hz, H-eight), 6.xc (1H, d, J = 8.0 Hz, H-3'), 7.65 (1H, dd, J = eight.0/1.viii, H-2'), 7.69 (1H, d, J = 1.viii Hz, H-half-dozen'). ESIMS m/z 610.16 [Yard]+, 611.xvi [Yard + H]+, 633.fifteen [M + Na] +, 609.xv [M-H]−.
Discussion
Medicinal plants are important sources of biologically active natural products which due to their curative properties accept been studied for many years [25, 26]. The present written report was designed to find the bactericidal potential of extracts of C. angustifolia. A. junii, S. mercescens, E. cloacae, P. aeroginosa, and S. typhi take been implicated in the pathogenesis of various infectious diseases [27]. Among the extracts, the methanol extract of C. angustifolia displayed the highest activity and a broad spectrum of activeness against pathogenic bacterial strains. It was reported that the bactericidal activeness was due to the presence of flavonoids establish in the methanol excerpt [28]. It was studied that methanol and ethanol extracts of C. angustifolia possess significant antibacterial activity confronting East. coli, Klebsiella pneumoniae and Shigella shinga [17]. The current findings suggest that methanol, ethanol and ethyl acetate extracts are rich in flavonoids which are responsible for antimicrobial activities. Flavonoids including rutin (3) have been reported to have antimicrobial activities against resistant bacterial strains [29]. The aqueous extract of C. angustifolia was non active at the highest concentrations tested against A. junni, Due east. cloacae and P. aeroginosa. It was reported that this was due to the lower extraction of antimicrobial compounds into the aqueous extract or to minimum availability of the aqueous extract to the microorganism [thirty]. It was also reported that the n-butanol extract of C. angustifolia showed maximum antibacterial potential against S. aureus and typhi with 13 and xv mm zones of inhibition, respectively [31]. The current report revealed that A.junii, S. mercescens and P. aeroginosa show resistance against the standard antibiotics amikacin and cefepime, while these bacterial strains showed sensitivity to C. angustifolia extracts. The electric current study as well showed that C. angustifolia exhibits potential fungicidal property against C. albicans. It was shown earlier that saponins, agile ingredients of C. angustifolia, were involved in antifungal activity against Colletotridium dematium [32]. It was reported before that a butanol extract of C. angustifolia exhibited antifungal activity against Aspergillus terrus, A. flavus and A. niger [31]. The antimicrobial activities of medicinal plants were due to the presence phytochemicals including saponins, terpenoids, flavonoids, phenolics, and alkaloids [33].
In the present written report, antioxidant activities of C. angustifolia extracts revealed that methanol, ethanol, ethyl acetate and acetone exhibited significantly higher scavenging percentages and are correlated by phenolic compounds. Phenolics and flavonoids are secondary metabolites derived from tyrosine and phenylalanine with potent antibacterial and antioxidant activities [34]. Information technology was reported that isolated flavonoids quercimeritrin (ane), scutellarein (two), and rutin (three) accept significant antioxidant activities against oxidative stress [35–37]. Note that all iii have one,2-dihydroxybenzene groups which are readily oxidized to orthoquinones, making them potent antioxidants. The aqueous excerpt showed a poor DPPH-scavenging activity of 67.seven %. This is because the flavonoids and phenols responsible for antioxidant activity are poorly extracted into the aqueous extract [38]. No detailed anticancer study of C. angustifolia has been reported before. This written report was carried out to evaluate the anticancer potential of C. angustifolia and it revealed that methanol and ethanol extracts of C. angustifolia exhibit anticancer properties. It was reported before that secondary metabolites like flavonoids tin can exist responsible for anticancer activities [29]. The current study supports the idea that the anticancer activity is due to the presence of isolated flavonoids, which were found in methanol and ethanol extracts of C. angustifolia. It was investigated earlier that scutellarien (ii), extracted from Scutellaria lateriflora, possesses anticancer activity by significantly suppressed the proliferation of HT1080 human fibrosarcoma cells through induction of apoptosis [39]. In a similar study, information technology was revealed from the in vivo experiment that that the size and weight of the tumor was reduced after treatment with scutellarein (2) [40]. On the other hand, rutin (3) has been reported as anticancer agent by inducing apoptosis and jail cell bicycle arrest in murine leukemia WEHI-three cells [41]. Information technology was also reported that rutin (3) had the potential to kill the chest cancer cells in MDA-MB-231 cell line [42]. Based on the findings, the current study supports the hypothesis that antimicrobial, anticancer and antioxidant activities of C. angustifolia are due to at least in role to the presence of isolated flavonoids.
Conclusions
The present written report was carried out to explore antimicrobial, antioxidant, and anticancer potential of C. angustifolia. Maximum antioxidant and anticancer activities were observed for methanol, ethanol and ethyl acetate extract which was correlated with phenolic and flavonoid contents. Bioactivity-guided screening of methanol, ethanol and ethyl acetate extracts revealed the presence of quercimeritrin (1), scutellarein (2), and rutin (three), all known to accept useful bioactivities including antimicrobial, antioxidant and anticancer activities. These findings shows the importance of screening medicinal plants for antimicrobial, anticancer and antioxidant agents against resistant bacterial strains, various cancers and degenerative diseases caused by oxidative stress.
Abbreviations
- BHA:
-
Butylated hydroxyanisole
- BHT:
-
Butylated hydroxytoluene
- DPPH:
-
1,1-diphenyl-ii-picrylhydrazyl
- ESI-MS:
-
Electronspray ionisation mass spectrometry
- FBS:
-
Fetal bovine serum
- GAE:
-
Gallic acrid equivalent
- HCEC:
-
Human corneal epithelial cells
- HeLa:
-
Man cervical cancer cell line
- Hep2:
-
Human epidermoid larynx carcinoma jail cell line
- HPLC-MS:
-
High pressure liquid chromatogropy mass spectrometry
- MCF-7:
-
Michigan cancer foundation-seven
- MTT:
-
3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium- bromide
- NMR:
-
Nucleic magnetic resonance
- PG:
-
Propyl gallate
- QE:
-
Quercetin equivalent
- rpm:
-
Revolution per minute
- RPMI-1640:
-
Roswell park memorial institute-1640
- TBH:
-
t-butylhydroxytoluene
References
-
Sharma R, Sharma CL, Kapoor B. Antibacterial resistance: electric current issues and possible solutions. Indian J Med Sci. 2005;59:120–9.
-
Djeussi ED, Noumedem AKJ, Seukep AJ, Fankam GA, Voukeng KI, Tankeo BS, Nkuete HLA, Kuete V. Antibacterial activities of selected edible plants extracts confronting multidrug-resistant Gram-negative leaner. BMC Complement Altern Med. 2013;13:one–eight.
-
Adedapo AA, Jimoh FO, Koduru Due south, Afolayan AJ, Masika PJ. Antibacterial and antioxidant backdrop of the methanol extracts of the leaves and stems of Calpurnia aurea. BMC Complement Altern Med. 2008;eight:53–seven.
-
Ashraf A, Sarfraz RA, Rashid MA, Shahid M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an of import medicinal establish (Euphorbia royleana) from Pakistan. J Nutrient Drug Anal. 2014;23(1):109–fifteen.
-
Ashraf A, Sarfraz RA, Mahmood A, Din MU. Chemical composition and in vitro antioxidant and antitumor activities of Eucalyptus camaldulensis Dehn. leaves. Ind Crop Prod. 2015;74:241–eight.
-
Qayyum A, Sarfraz RA, Ashraf A, Adil S. Phenolic composition and biological (anti diabetic and antioxidant) activities of different solvent extracts of an endemic plant (Helitropium strigosum). J Chil Chem Soc. 2016;61(1):2642–v.
-
Saeed N, Khan RM, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla 50. BMC Complement Altern Med. 2012;12:221–33.
-
Aslanturk Bone, Celik TA. Antioxidant, cytotoxic and apoptotic activities of extracts from medicinal establish Euphorbia platyphyllos 50. J Med Plants Res. 2013;vii:1293–304.
-
Cai Y, Luo Q, Dominicus Chiliad, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with cancer. Life Sci. 2004;74:2157–84.
-
Art IC, Hollman PC. Polyphenols and disease risk in epidemiological studies. Am J Clin Nutr. 2005;81:317–25.
-
Alpinar K, Ozyurek Grand, Kolak U, Guclu One thousand, Aras C, Altun Thou, Celik SE, Berker KI, Bektasoglu B, Apak R. Antioxidant Capacities of some food plants wildly grown in ayvalik of turkey. Nutrient Sci Tech Res. 2009;fifteen:59–64.
-
Sherwin FR. Antioxidant. In: Branen R, editor. Food additive biological properties. New York: Marcel Dekker; 1990.
-
Kumar GP, Singh SB. Antibacterial and antioxidant activities of ethanol extracts from trana himalayan medicinal plants. Eur J Appl Sci. 2011;3:53–vii.
-
Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, Bedi YS, Taneja SC, Bhat HK. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2007;9:581.
-
Elkady AI, Osama AA, Baeshen NA, Rahmy TR. Differential Control of Growth, Apoptotic activity, and gene expression in human breast cancer cells past extracts derived from medicinal herbs Zingiber officinale. J Biomed Biotechnol. 2012;2012:6143.
-
Laghari AQ, Shahabuddin M, Nelofar A, Laghari AH. Extraction, identification and antioxidative properties of the rich fractions from leaves and flowers of Cassia angustifolia. Am J Anal Chem. 2011;2:871–8.
-
Bameri Z, Negar AB, Saeide S, Saphora B. Antibacterial activity of C. angustifolia excerpt against some human pathogenic leaner. J Nov Appl Sci. 2013;ii:584–half-dozen.
-
Murray PR, Baroon EJ, Pfaller MA, Tenover FC, Yolke RH. Transmission of clinical microbiology. 6th ed. Washington DC: American Guild for Microbiology; 1995.
-
Mohamed AA, Ali SI, El-Baz FK. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygiumcumini leaves. PLoS One. 2013;8:e60269.
-
Mosmann T. Rapid colorimetric analysis for cellular growth and survival: awarding to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
-
Harborne JB. Phytochemicals methods. London: Chapman and Hall Ltd; 1973.
-
Trease GE, Evans WC. Pharmacognosy. 11th ed. London: Bailliere Tindall; 1989. p. 45–50.
-
Sofowra A. Medicinal plants and traditional medicine in Africa. Ibadan: Spectrum Books Ltd; 1993. p. 191–289.
-
Khatoon M, Ekramul I, Rafikul I, Rahman AA, Khurshid AHMA, Khondkar P, Rashid M, Parvin S. Estimation of total phenol and in vitro antioxidant activity of Albizi aprocera leaves. BMC Res Notes. 2013;half dozen:121.
-
Ahmed N, Mahmood A, Ashraf A, Bano A, Tahir SS, Mahmood A. Ethnopharmacological relevance of indigenous medicinal plants from district Bahawalnagar, Punjab, Pakistan. J Ethnopharmacol. 2015;175:109–23.
-
Ahmed N, Mahmood A, Tahir SS, Bano A, Malik RN, Hassan Due south, Ashraf A. Ethnomedicinal knowledge and relative importance of indigenous medicinal plants of Cholistan desert, Punjab Province, Pakistan. J Ethnopharmacol. 2014;155:1263–75.
-
Sanches IS, Saraiva ZC, Tendeir TC, Serra JM, Dias DC, Delencastre H. Extensive Intra-Hospital spread of methicillin resistant Staphyloccocal clone. Int J Infect Dis. 1998;3:26–31.
-
Das M, Tiwari RKS, Shrivastava DK. Techniques for evaluation of medicinal plant products as antimicrobial agent: Electric current methods and futurity trends. J Med Plants Res. 2010;4:104–11.
-
Priya B, Anil KS. Anti-cancer potential of flavonoids: contempo trends and future perspectives. Biotech. 2013;3:439–59.
-
Ishtiaq S, Ashraf K, Hayat MQ. Phytochemical analysis of Nigella Sativa L. and its antibacterial activity against clinical isolates identified past Ribotyping. Int J Agri Biol. 2013;15:1151–6.
-
Gnanavel South, Bharathidasan R, Mahalingam R, Madhanraj P, Panneerselvam A. Antimicrobial Activeness of Strychnosnuxvomica Linn and C. angustifolia Linn. Asian J Pharm Tech. 2012;2:08–11.
-
Khan NA, Srivastava A. Antifungal activity of bioactive triterpenoid saponin from the seeds of Cassia angustifolia. Nat Prod Res. 2009;23:1128–33.
-
Cooper J, Niggli U, Leifert C. Handbook of organic food safety and quality. England: Abington Hall; 2006.
-
Montoro P, Braca A, Pizza C, Tommasi ND. Structure-antioxidant activeness relationships of isolated from unlike plant species. Food Chem. 2005;92:349–55.
-
Siraichi JTG, Felipe DF, Brambilla LZS, Gatto MJ, Terra VA. Antioxidant capacity of the leaf extract obtained from Arrabidaea chica cultivated in southern Brazil. PLoS Ane. 2013;8:e72733.
-
Jianxiong YB, Juan G, Jiangfeng Y. In vitro antioxidant backdrop of rutin. Food Sci Technol. 2008;41:1060–6.
-
Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I. Study on the inhibitory result of tannins and flavonoids against 1,one-diphenyl-2-picrylhydrazyl radical. Biochem Pharmacol. 1998;56:213–22.
-
Ao C, Li A, Elzaawely AA, Xuan DT, Twata S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa Fifty. fill extract. Food Control. 2008;nineteen:940–viii.
-
Didem DO, Berrin O, Selda O, Fatma E. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol Res. 2010;165:496–504.
-
Xiujuan S, Guangfeng C, Xiaoqiang L, Yu Q, Shuzhang Y, Yan Z, Xuexun F, Chen Z, Xiaoqing 50. Scutellarein inhibits cancer cell metastasis in vitro and attenuates the development of fibrosarcoma in vivo. Int J Mol Med. 2015;35:31–8.
-
Lin JP, Yang JS, Lin JJ, Lai KC, Lu HF, Ma CY, Sai-Chuen WR, Wu KC, Chueh FS, Gibson WW, Chung JG. Rutin inhibits man leukemia tumor growth in a murine xenograft model in vivo. Environ Toxicol. 2012;27:480–4.
-
Aliye AP, Iryna SM, Yusuf CG, Kadir B, Mevzule Y, Sundas F, Ammad AF. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Jail cell Int. 2014;fourteen:124.
Acknowledgements
The Pakistan Establish of Medical Sciences Hospital, Islamabad, Pakistan, provided bacterial strains. Dr. Hajra Sadia provided HeLa cell lines and Muhammad Imran provided cell culture expertise. Alia sadiq for providing her intellectual support.
Funding
This enquiry was supported by Research Advisers, National University of Sciences and Technology (NUST), H-12 Islamabad, Pakistan.
Availability of data and materials
The datasets during and/or analysed during the current study bachelor from the corresponding author on reasonable request.
Authors' contributions
SIA designed and performed experiments, assembled the data and wrote the manuscript. MQH supervised all the experimental work and edited the manuscript. MT supervised the experimental work, gave the technical and conceptual advice. QM designed and help in performing the anticancer activity. MI participated in designing and provide the facilities, chemicals & cell lines to perform the anticancer action. KK provide technical expertise in performing preparative HPLC. RBB edited and canonical the concluding version of manuscript. All authors read and approved the last manuscript.
Competing interests
There are no competing interest between authors associated with this publication.
Consent for publication
Not Applicable.
Ethics approving and consent to participate
Non Applicable.
Writer information
Affiliations
Corresponding writer
Rights and permissions
Open Access This article is distributed under the terms of the Creative Eatables Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted utilise, distribution, and reproduction in any medium, provided yous give appropriate credit to the original author(south) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zip/1.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this commodity
Ahmed, S.I., Hayat, M.Q., Tahir, M. et al. Pharmacologically agile flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement Altern Med 16, 460 (2016). https://doi.org/ten.1186/s12906-016-1443-z
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s12906-016-1443-z
Keywords
- Cassia angustifolia
- Antimicrobial
- Antioxidant
- Anticancer
- Flavonoids
Source: https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-016-1443-z
0 Response to "Anticancer, Antioxidant and Antimicrobial Activity of Annonaceae Family"
Postar um comentário